Rhizaria
Back to main indexJump to section:
Introduction
The last major lineage of SAR, Diaphoretickes and eukaryotes is that of Rhizaria. Defined primarily by molecular analyzes, it brings together a motley collection of organisms that have long remained without any affiliation (Figure 331). It is probable that in the near future other mysterious organisms will join this collection. The relatedness of the different lines is confirmed by the presence of a signature in the gene encoding ubiquitin, a protein targeting other proteins that are to be degraded by the proteasome. Ubiquitin is typically encoded as a polyprotein which is cleaved at a glycine residue. In Rhizaria, glycine is followed by a characteristic insertion of one or two amino acids, suggesting a particular maturation mechanism. This insertion is not, however, present in all lineages.
Often the different taxa of Rhizaria are only known by a few species. However, this super-branch is far less explored than the others, and data obtained from environmental DNA analyzes show that its biodiversity is very poorly appreciated. For example, Vampyrellida amoebae are currently only known by a few dozen species. Yet their diversity could be as great as that of Eumycota! Likewise, because of their great divergence, the sequences do not always make it possible to precisely define the links of kinship between the different groups. We therefore await a global phylogenetic analysis based on a sufficient number of species to confirm the phylogenetic tree of Rhizaria and to define the different branches/classes. Most of the members have a phagotrophic stage that is amoeboid in shape and capable of forming filopodia or reticulopods. In most cases, these extensions are reinforced by microtubules. Some species are photosynthetic. Such lineages result either from a secondary endosymbiosis with a Viridiplantae (Figure 39 and Table 5), or from a primary endosymbiosis independent of that which led to all the other photosynthetic eukaryotes (Box 6). Few species are parasitic, in particular no species parasitizes humans, which partly explains the lack of studies on these organisms. The vast majority of these organisms are unicellular. However, a few have evolved a simple aggregative or non-aggregative multicellularity. Many species form plasmodia (multinucleate cytoplasm without compartments), which can reach large sizes, and some show specializations in different parts of the thallus. Certain groups are able to surround themselves with calcareous thecae or siliceous skeletons. Note that the main and among the oldest indisputable eukaryotic micro-fossils belong to the Rhizaria and that our knowledge of the earth’s past is therefore largely based on data acquired on this group.
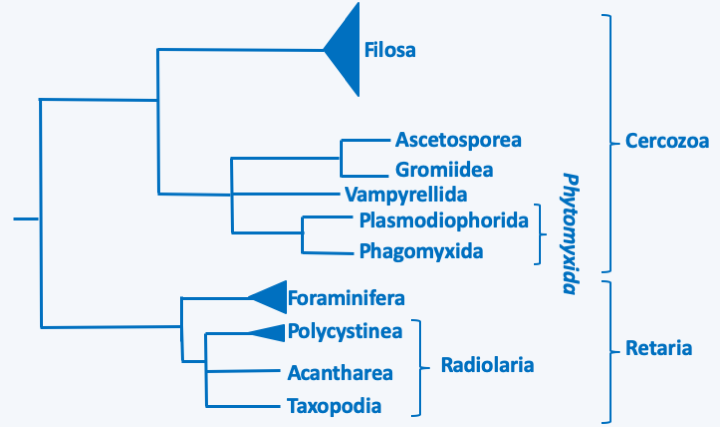
Figure 331.
Phylogeny of Rhizaria.Cercozoa
The Rhizaria super-branch is currently divided into two branches, Cercozoa and Retaria (Figure 331).
Plasmodiophorida & Phagomyxida
One of the lineages that seems to diverge first is the Phytomyxida superclass (Figure 331), of which a few dozen species have been described. They are obligate intracellular parasites of plants, Ochrophyta algae or Oomycota water molds. Two classes are defined, that of Plasmodiophorida which attacks terrestrial organisms and that of Phagomyxida which parasitizes marine organisms (Figure 331). For a long time, these two lineages were also defined by their hosts, the first attacking plants and the second algae, but the discovery that Woronina pythii, a pathogen of Oomycota, is a Plasmodiophorida and that Plasmodiophora diplanthereae which attacks plants is in fact a Phagomyxida has made this criterion obsolete. These organisms have an intracellular plasmodium in trophic form, which for a long time assimilated them with the obligate biotrophic fungi. Mitosis is unique in this group because it presents cross-shaped figures, hence the name cruciform mitosis (Figure 332). Phytomyxida disperse with the help of biflagellate zoospores. These enter their host using a characteristic method (Figure 333).
Figure 332.
Cruciform mitosis in Plasmodiophora brassicae.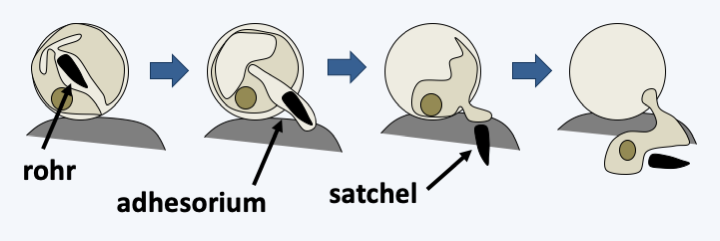
Figure 333.
Penetration method used by Plasmodiophora brassicae to penetrate its host. The zoospore encysts before entering. The cyst has a tubular structure or rohr containing a dagger-shaped body called a satchel. Penetration begins with the attachment of the zoospore with an adhesive factor that forms an adhesorium. After attachment, the satchel and the contents of the zoospore are injected through the adhesorium and cell membrane, into the cytoplasm of the host cell.Some species of agronomic importance include Plasmodiophora brassicae, a parasite of Brassicaceae including cabbage and rapeseed (Figure 334), and Spongospora subterranea, which infects potatoes. At the plant level, the infection leads to enlargement and the formation of tumors. Funnily enough, this led some doctors at the turn of the 20th century to think that human cancers could be caused by similar organisms! Polymyxa attack many plants including sugar beets. On their own, they do not cause visible symptoms, but serve as vectors for some plant viruses. These pests can cause serious damage to crops. Especially since the spores of these organisms can last a long time in the soil: up to 10 years for Plasmodiophora brassicae, up to 8 years for Spongospora subterranea and Polymyxa graminis. There is no effective treatment other than to drain the soil well to avoid the propagation of zoospores, the application of lime to increase the pH of the soil and especially the rotation of crops, by leaving a sufficiently long time between two species susceptible to pests.
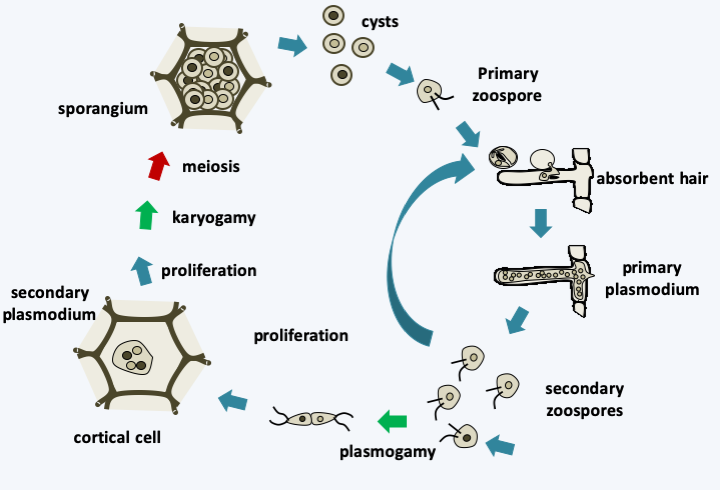
Figure 334.
Plasmodiophora brassicae cycle. In the spring, when roots are susceptible to infection, resting cysts germinate and produce biflagellate and uninuclate primary zoospores that swim in water trapped in the soil. Infections are therefore particularly severe during rainy years. The pathogen readily enters through root hairs and sometimes through root wounds. Inside the root hair, the zoospore develops into a small plasmodium which then differentiates into uninucleated sporangia. From these sporangia emerge secondary biflagellate zoospores which will leave their host or reinfect neighboring cells of the root cortex. They can also serve as gametes, fuse and re-infect a new host. The resulting miniplasmodes will proliferate and infect neighboring cells. This leads to cell hypertrophy and the formation of root tumors. During these proliferative cycles karyogamy and meiosis occur to give sporangia containing cysts which will differentiate into primary zoospores.Vampyrellida
Vampyrellida currently includes a few dozen species, but metagenomics shows that these organisms are very diverse and ubiquitous in soils as well as freshwater and especially marine sediments. Their trophic stage is naked amoebae with filiform (threadlike) pseudopods, whose mode of nutrition is original (Figure 335). Indeed, these trophozoites pierce the cell walls of their prey, various algae or yeasts and molds, and suck their contents, like a vampire. Some may, however, swallow their prey completely or penetrate inside and devour its contents. Most species have the potential to produce large plasmodia which result from the fusion of trophozoites. These plasmodia can fuse with each other and reach sizes greater than a millimeter. They can also differentiate several types of cysts, including those involved in digestion after ingestion of prey, or persistent cysts.
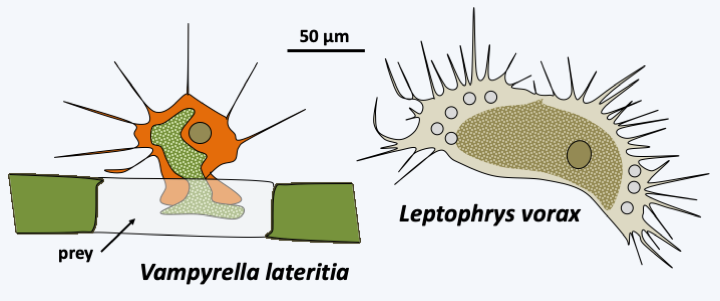
Figure 335.
Diversity of Vampyrellida.Filosa
The Filosa sub-branch includes a large set of taxa with the status of class, sub-class, order, super- or sub-order… This set is a relic of a classification of Cercozoa in two Filosa sub-branches and Endomyxa, as well as in several orphan lines, some of which have been grouped into the class of Granofilosea. These sub-branches and class are probably polyphyletic and are in the process of being dismembered. This work is almost finished for that of the Endomyxa which seem to be divided into two lineages, one containing the Phytomyxea, the other regrouping the Ascetosporea and related lineages (Figure 331). At present, it is therefore not clear whether the Filosa are mono-, poly- or paraphyletic, especially since orphan lines seem to have to join this sub-branch, their inclusions each time modifying the phylogenetic tree… As with other groups of Rhizaria, the genetic diversity of the different lineages is important, but, in most cases, it is only understood by sequencing data of DNA extracted directly from the environment. Filosa probably have major ecological roles; some, such as Glissomonadida, for example, are the main consumers of bacteria in the soil. The Filosa (Figure 336) have very variable trophic forms: naked amoeba with phagotrophic or photosynthetic filopodia, amoeba with thecae or with phagotrophic or photosynthetic scales, radiolar or heliozo-type amoeba, even with distinctive cytoskeletal structures such as siliceous bars; others are small flagellates or amoeboflagellates, naked or protected by scales… For most species, the biology is poorly known, in particular our knowledge of life cycles is often fragmentary because these organisms are not easily cultivated in the lab. Photosynthetic species have two distinct origins. Chlorarachniophyta result from the secondary endosymbiosis of a Viridiplantae (Figure 39 and Table 5). These amoeboflagellates have retained a nucleomorph (Box 8) and are still capable of phagocytizing prey. The Paulinella acquired their plastid via independent endosymbiosis (Table 5 and Box 6). Species of the genus Guttulinopsis evolved an aggregative multicellularity (Figure 337). These amoeba, unlike the majority of Rhizaria, have lobed pseudopods, once again showing the evolutionary plasticity of the “Filosa”.
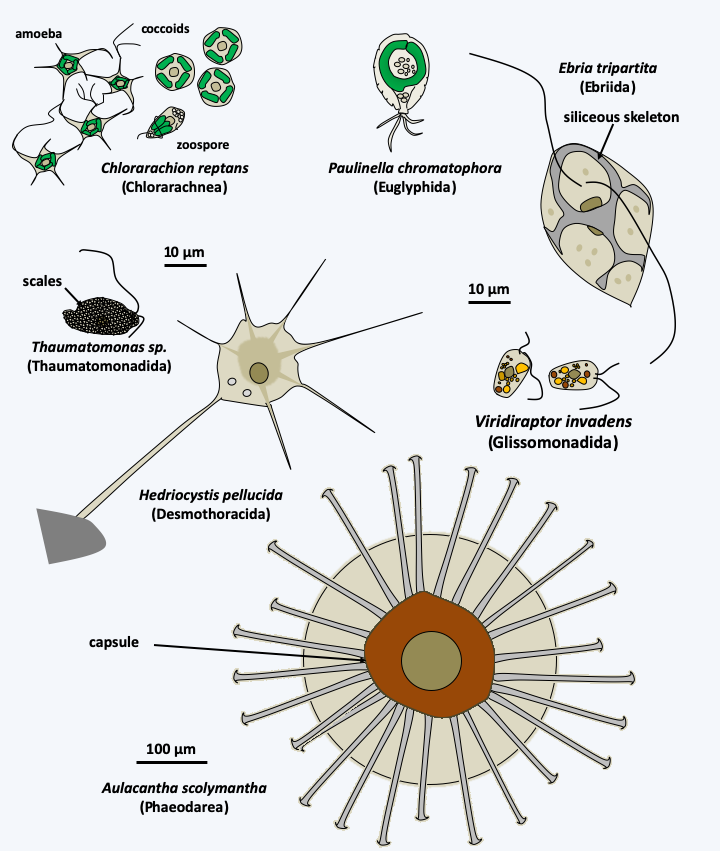
Figure 336.
Diversity of the Filosa.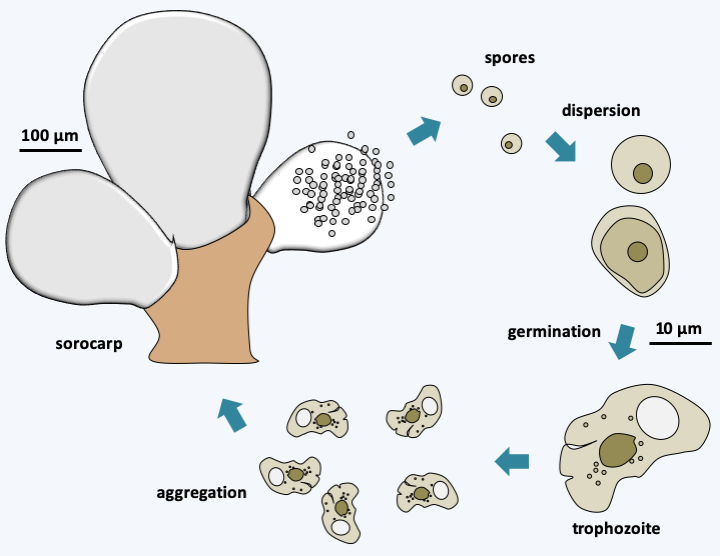
Figure 337.
Cycle of Guttulinopsis vulgaris.Ascetosporea, Gromiida and Mikrocytos
The last lineage of Cercozoa, grouping together Ascetosporea and Gromiida, is probably monophyletic. Ascetosporea are parasites of aquatic invertebrates, including many shellfish farmed in aquaculture. There are two groups, the Haplosporidia which are intracellular and the Paramyxida which are extracellular and live in the digestive cavities of their hosts. Their life cycles (Figure 338) seem complex and poorly understood, but involve the differentiation of plasmodia and/or spores in some of the known species. The Gromiida are free species whose trophic stage consists of a giant, multinucleated plasmodium, protected by an iron-rich protein test (shell), spherical or ovoid and pierced with one or more openings (Figure 339). From these openings emerge filiform pseudopods. Gromiida can grow to sizes of several centimeters. They live on the seabed, sometimes at very great depth, where they move by dragging or rolling and leave characteristic traces. Similar fossil traces have in the past been attributed to small primitive animals… Mikrocytos mackini is an intracellular parasite of oysters (Figure 340). Molecular phylogenies have great difficulty in resolving its phylogenetic position because its genome evolves very rapidly. It probably derives from Haplosporidia by regressive evolution. Indeed, this organism lacks mitochondria, but probably has a mitosome involved only in the assembly of “iron-sulfur” complexes. A related genus, Paramikrocytos, parasitizes crabs.
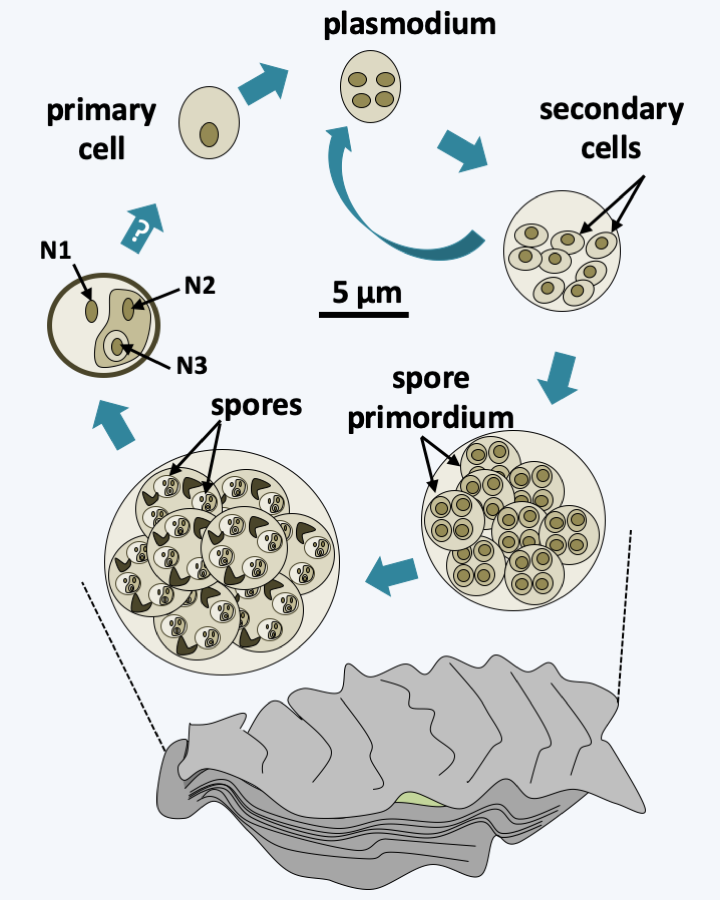
Figure 338.
Life cycle of Marteilia refringens. This species belonging to Paramyxea is an extracellular parasite of the oysters Ostrea edulis. The postulated cycle begins with the presence of so-called primary cells mainly within the digestive epithelium and gills. These cells proliferate, possibly schizogonically, and invade the host. Then, the secondary cells differentiate into primordia, which will internally sporulate spores. The spores themselves are made up of three cells nested within each other (see nuclei N1, N2 and N3). How spores return primary cells, as well as the locations of fertilization and meiosis, are still subject to conjecture. It is possible that there is a second host in which Marteilia refringens completes its cycle.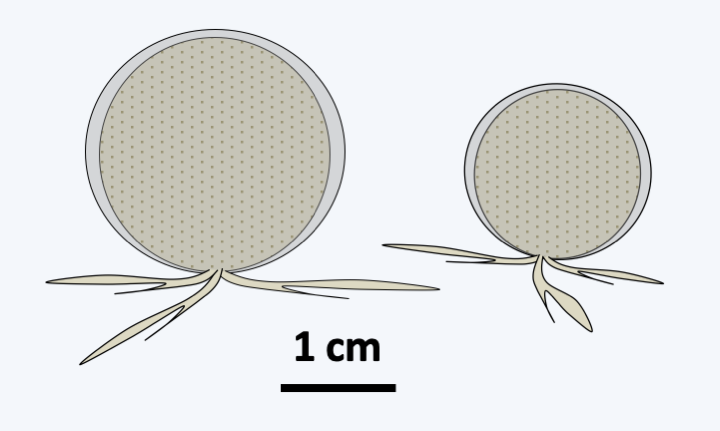
Figure 339.
The Gromiida Gromia sphaerica.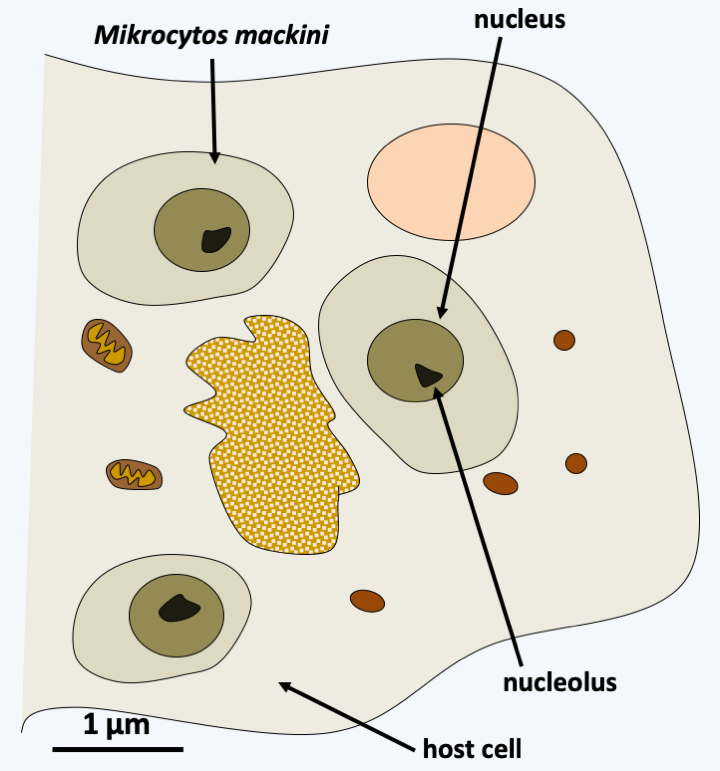
Figure 340.
Mikrocytos mackini, parasite of the oyster Crassostrea gigas.Retaria
The Retaria phylum includes amoeboid phagotrophic organisms with long and thin, more or less reticulated pseudopodia. Traditionally, two classes were defined. That of the “Radiolaria” included organisms in the form of the sun, like the heliozoans, but which had an internal delineation in ectoplasm and endoplasm. Their internal cytoskeleton was composed of radiating spicules of variable composition, though not calcareous. That of Foraminifera included organisms possessing reticulated pseudopods and for the most part possessing an organic test, calcified in many species. A part of the old Radiolaria, now classified in the class Phaeodarea (Figure 336), left the group because these organisms are in fact Cercozoa related to the Filosa.
Taxopodia
The lineage of Retaria which seems to diverge first, that of Taxopodia, contains only one described species: Sticholonche zanclea (Figure 341). This large marine protozoan has a heliozo-like structure because it does not have a central capsule. Molecular phylogenies clearly place it within the Retaria, but its exact position is not clearly defined. It is either placed at the base of the tree as inFigure 331or as a sister group of Acantharea with which it shares some ultrastructural characters, such as the presence of contractile fibers.
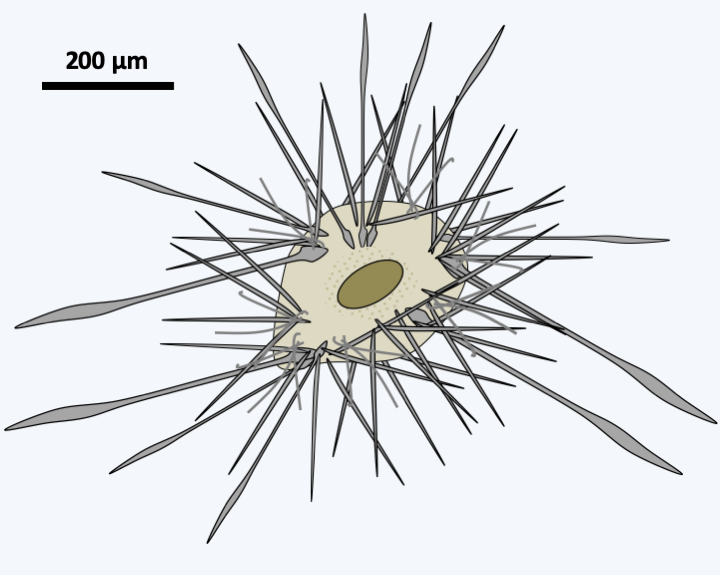
Figure 341.
Sticholonche zanclea.Polycystinea
The Polycystinea radiolarians are planktonic, passive, isolated or colonial microorganisms whose size varies from a few tens of microns to more than one mm and which inhabit all the seas of the globe (Figure 342). There are about 3,000 species. They are capable of emitting multiple radiating cytoplasmic extensions, axopods, filopodia and reticulopods. They are characterized in most species by the presence of an intracytoplasmic siliceous skeleton made up of SiO2. The skeleton adopts various forms, often with a radial or bilateral symmetry (Figure 342). It is mainly localized in the ectoplasmic part where it is accompanied by digestive vacuoles and sometimes by zooxanthellae or endosymbiotic chlorellae (Figure 343). The external part also contains alveoli resembling bubbles that might help with flotation. The endoplasm contains the rest of the cell body with one or more nuclei and the entire nucleus/reticulum/golgi network, as well as the mitochondria. The capsule is made up of chitinous material with pores called “fusules”. Little is known about the reproduction of Polycystinea. Cell division takes place by binary fission, multiple fission or budding. Cells from schizogonia are small, pyriform and biflagellate. They contain a strontium-rich crystal, an element found in the skeleton of Acantharea, the other group of radiolarians. Mitoses are closed. Sexual reproduction is not known. Polycystinea are very useful to paleontologists because there are many fossils of their skeletons which are used for stratigraphic dating. These organisms were already present 600 million years ago.
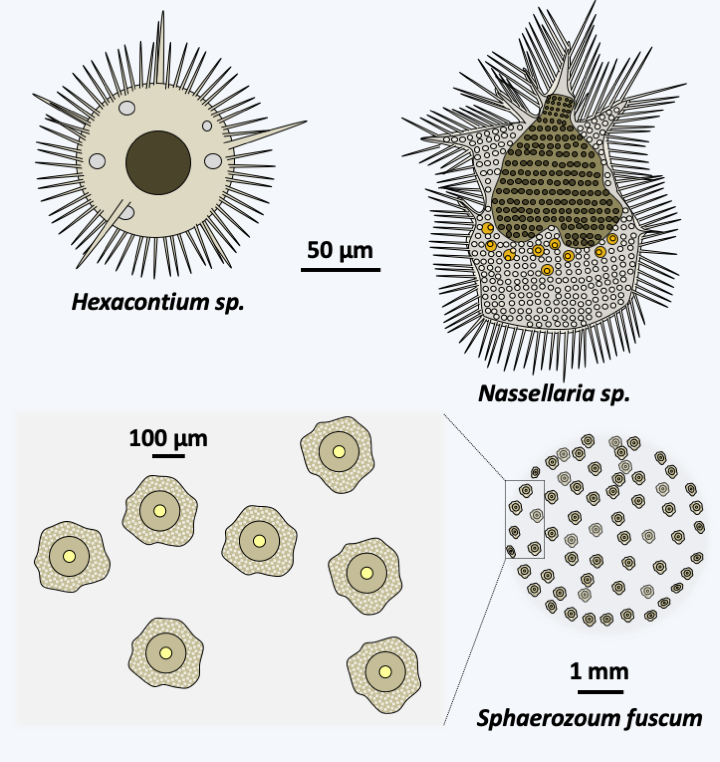
Figure 342.
Diversity of Polycystinea.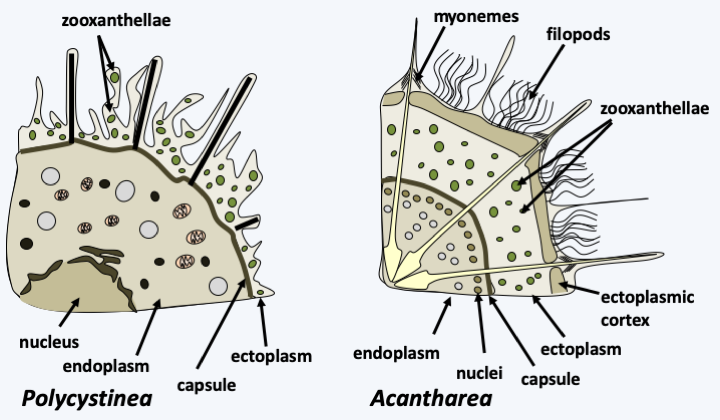
Figure 343.
Cell structure in radiolarians.Acantharea
The Acantharea class contains about 150 species (Figure 344). They are planktonic marine unicellular organisms that are mainly found in warm waters. They measure from 20 to 800 μm. Their cells have the same organization as Polycystinea, including the presence of endosymbionts (Figure 343). However, the central capsule does not have the same structure as in Polycystinea because it is devoid of fusules. Acantharea are characterized by the presence of twenty radiating spicules which meet in the center of the cell. Their position is fixed but their shapes very variable. They are composed of strontium sulfate, a mineral that does not fossilize. The outermost part of the spicules is associated with myonemes, which are protein fibers capable of retraction, but differ in composition from actomyosin fibers of muscles in animals. They are probably involved in the flotation of Acantharea. We do not yet know exactly the life cycles of these organisms but it seems that they are capable of sexual reproduction.

Figure 343.
Cell structure in radiolarians.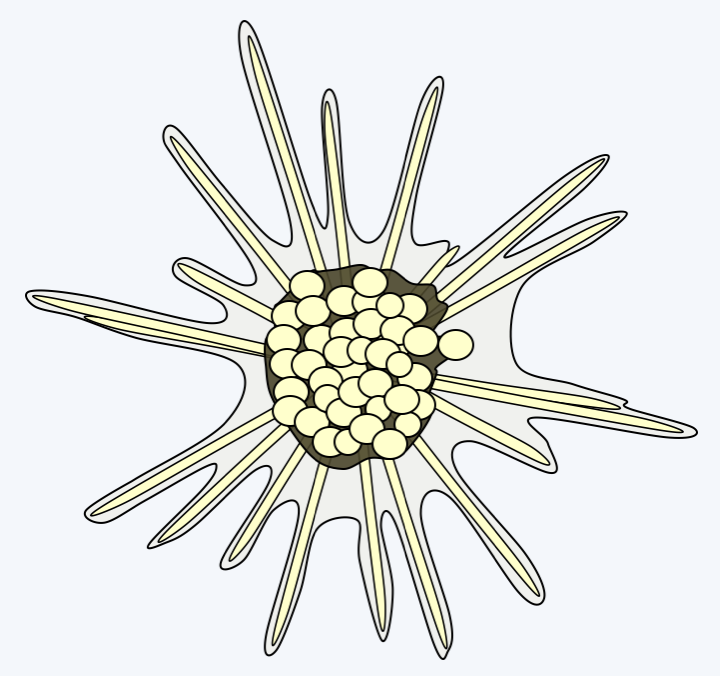
Figure 344.
Gigartacon fragilis, an Ancantharea.Foraminifera
The last group of Rhizaria is that of Foraminifera. It is currently represented by about 4,000 species, making it the most species-rich taxon of Rhizaria, and many fossil species are known from it. As with other Rhizaria, environmental DNA analyzes show a great genetic diversity of which only a small part is currently known from morphologically described species. Most are benthic and inhabit the ocean floor; some are planktonic. Many live in fresh water or in the soil. These unicellular organisms are amoeboid cells surrounded by a test of varying composition and shape (Figure 345). Indeed, they can result from the agglutination of more or less specific particles, crystals or microbeads of calcite and produce a single chamber or lodge in the case of so-called unilocular species, several lodges for multilocular species; these compartments can be globular or tubular (Figure 346). Nevertheless, there are species without test. The glycoprotein test grows by addition of “compartments” and can subsequently become loaded with calcium and detrital material. The organism is made up of a single cell with one or usually several nuclei. The organism occupies one or more of the lodges it has made. Specializations between the different compartments are observed with, for example in certain large species, the presence of symbiotic algae in the internal compartments while the external compartments are more devoted to nutrition or reproduction. The compartments are pierced with pores through which filaments emerge, similar to the pseudopodia of amoebae, except that they are very fine and supported by microtubules. They have the ability to anastomose to form a network traversed by the flow of cytoplasm. For this reason they are called reticulopods. They are used to distribute food in the form of dissolved molecules, plankton or even small animals. When its growth is complete, the plasmodium-like cell measures, depending on the species, between 100 μm to more than 20 cm, as in Xenophyophorea, which form structures that resemble corals (Figure 345). In the latter case, the nuclei are then counted in billions per cell! Traditionally, Foraminifera were classified according to the presence, composition and form of their test. Molecular phylogenies (Figure 347) indicate that the ancestral species were probably naked. Unilocular tests have evolved repeatedly, rendering the majority of traditional orders/families obsolete. All of these unilocular lineages are currently included in the informal paraphyletic class of “Monothalamides”. Multilocularity seems to have occurred three times during the evolution of Foraminifera (Figure 347). Species forming globular chamber tests form two monophyletic lines. One, now identified as the class Globothalamea, includes the majority of species, the other containing only species belonging to the order Lagenida. Species having a tubular test form a monophyletic line, the class Tubothalamea. Note that test calcification appeared convergently at least five times independently in the three multilocular lineages, rendering previous classifications based on this criterion obsolete.
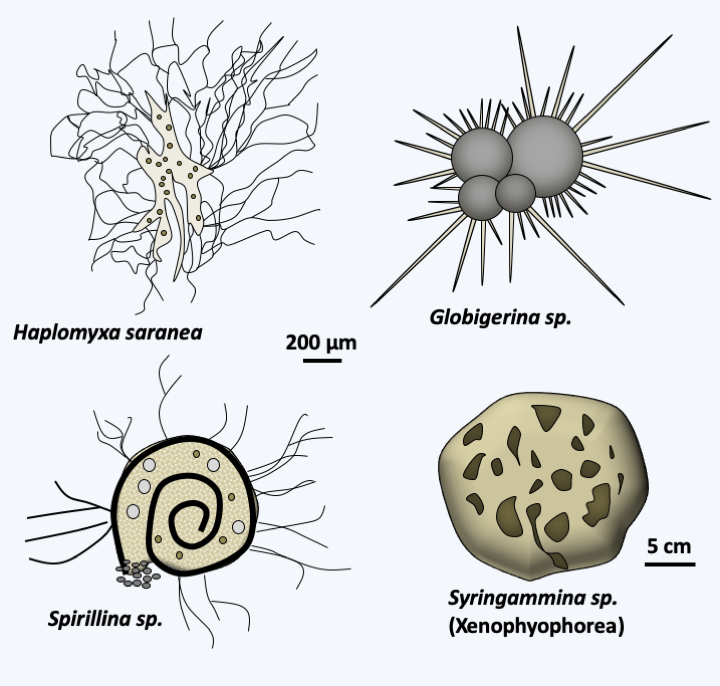
Figure 345.
Some Foraminifera.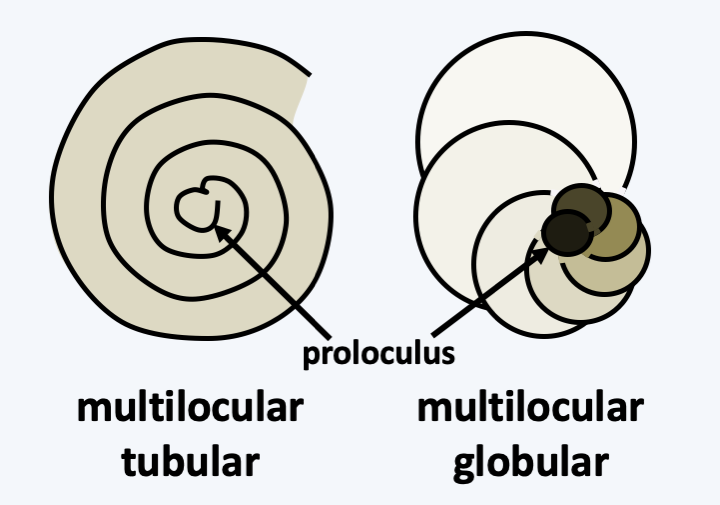
Figure 346.
Housing structure (test) in multilocular Foraminifera.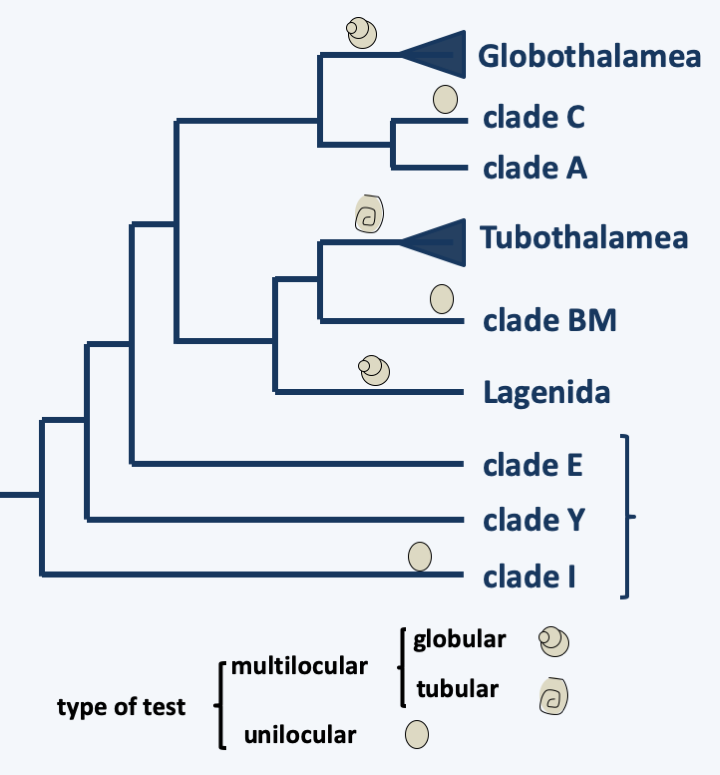
Figure 347.
Phylogeny of Foraminifera.Some species of Foraminifera are cosmopolitan while others have restricted habitats. Foraminifera can be present in significant quantities, up to several tens of thousands per m2 of seabed for some benthic species and they are used as food for many other organisms such as molluscs, fish, etc. These organisms are extremely abundant on the ocean floor, particularly in the deep sea where they appear to represent the dominant “fauna”. Their ability to breathe nitrate allows them to colonize environments poor in oxygen in particular. They are particularly abundant around the Antarctic but are nevertheless ubiquitous all around the globe. They move on the bottom leaving characteristic traces, like the Gromiida. Similar fossil traces have been found in sediments; these fossils suggest that Foraminifera have been an important component of “fauna” since the Precambrian.
Foraminifera reproduce asexually by multiple fissions from an initial individual. Individuals emerge completely differentiated after fragmentation of the initial plasmodium (schizogony). They have haplodiplobiontic sexual cycles with haploid and diploid phases showing sometimes very different morphologies that can lead to organisms of the same species looking very different (Figure 348).
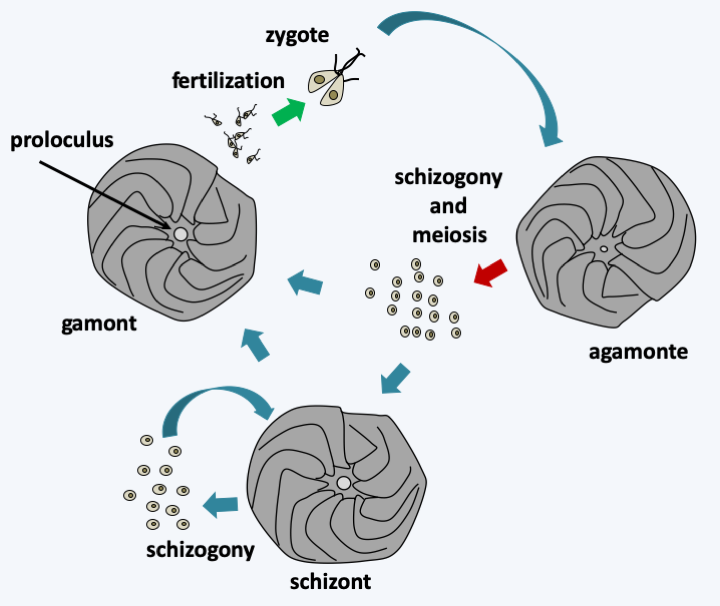
Figure 348.
Amphistegina gibbosa life cycle. In this species, the cycle is trigenetic with a diploid phase called agamont characterized by a small proloculus. The proloculus is the initial compartment from which the test is built. Schizogony followed by meiosis leads to the formation either of schizonts or directly of gamonts producing heterochontic biflagellate gametes. Schizonts have the possibility of giving back new schizonts after schizogony. They can also mature in gamontes. Schizonts and haploid gamonts are characterized by a large proloculus. Fertilization seems to be able to occur between gametes from the same gamont to give back a diploid agamont. It is not clear whether all Foraminifera species go through all stages of this cycle. Likewise, the ploidy of schizonts is not fully established. In Amphistegina gibbosa, the size of the proloculus suggests that they are haploidThe accumulation of limestone tests plays a significant part in the formation of marine sediments. They are found fossilized in very large quantities and variety, with more than 36,000 fossil species. These fossils show that Foraminifera were already present 500 million years ago. Foraminifera are widely used by paleontologists and geologists. Indeed, the structure of the test of the fossils present in the sediments makes it possible to determine the current species to which they are related. By extrapolating from the lifestyles of current species, it is thus possible to obtain information on the environment in which the sediment was formed. Analysis of the chemical composition of the tests also provides information on climatic conditions. In particular, sea temperature influences the oxygen isotopes present in the test because light isotopes evaporate more easily in warm seas.
Back to chapter index