Hacrobia
Back to main indexJump to section:
Introduction
The Hacrobia super-phylum is now known to be non-monophyletic. It previously grouped together several lineages whose evolutionary affinities were unclear (Figure 247). Most lineages contain phagotrophs, either flagellates or amoeboids. Others include algae from secondary endosymbiosis. In most of these algae, the ability to phagocyte prey has not been lost and they therefore have a mixotrophic strategy of nutrition. Some are major components of phytoplankton. The molecular phylogenies indicate, depending on the genes chosen, either monophyly or polyphyly of the group. Few shared characters support monophyly. In most, but not all lineages, the structure of the base of the flagellar apparatus is similar. In groups with plastids, these have some common characteristics, such as replacement by horizontal transfer of the plastid ribosomal protein rpl36 gene. As molecular phylogenies define two independent lineages (Figure 247), each containing algae, the history of the group is likely complex. The two scenarios considered under the monophyly hypothesis are firstly a single endosymbiosis, which implies five independent plastid losses. Alternatively, and more likely, two independent endosymbioses from the same photosynthetic precursor may have occurred. Regardless, the plastid is clearly originally derived from a Rhodophyta algae. What is therefore less clear is how it came to be in the photosynthetic lineages of Haptista and Cryptista.
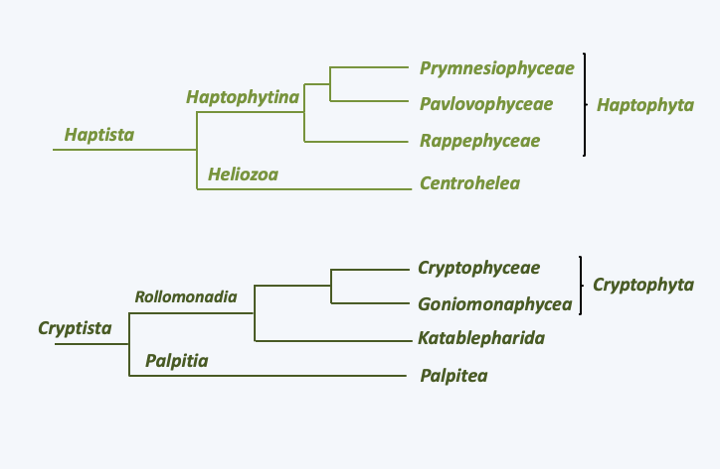
Figure 247.
Phylogeny of Haptista and Cryptista. The two groups were previously thought to form a monophyletic group called Hacrobia.Cryptista
The first major lineage, the Cryptista, contains a set of unicellular phagotrophs that are often very small (Figure 248). Some take only a flagellated form. This is the case in Palpitia, where currently only one species is known (Palpitomonas bilix), of Katablepharida where ten species are known, and Goniomonaphyceae which is known by four species of Goniomonas. These species are only the “tip of the iceberg” as environmental DNA analyzes show that the organisms belonging to these lineages are very diverse and abundant in marine and fresh waters. The preys of these organisms are diverse. Palpitomonas bilix and Goniomonas feed on bacteria, and Katablepharida on eukaryotes. The latter have a particular cytopharynx positioned at the front of the cell (Figure 248). One species, Hatena arenicola, does not differentiate this pharynx until after division that involves an uneven distribution of a photosynthetic endosymbiont. Indeed, this species began the establishment of an endosymbiosis with a Viridiplantae Prasinophyta of the genus Nephroselmis (Figure 45). Microhelida are only known from one species, Microheliella maris, which lives only as an amoeba with radiating pseudopodia. It was previously included among the “heliozoans” which included the set of amoebae with fine and radiating pseudopodia, conferring a sun shape. Their pseudopodia are often subtended by microtubules and are called axopods. This ancient class has been shown to be polyphyletic, and the term heliozoan, which no longer has any phylogenetic value, now characterizes sun-shaped amoebae. Note that the Heliozoa phylum has recently been reused to classify organisms related to Centrohelea, the class that contains the majority of heliozoan amoebae. This class belongs to the Haptista, the other major group of Hacrobia (Figure 247). The last Cryptista phagotrophic line, the Heliomonadida, is provisionally placed near the Microhelida on the basis of ultrastructural characteristics because no DNA sequence is available to confirm or reject this position. The best known species, Heliomorpha mucosa, also called Dimorpha mutans, alternates between a biflagellate form and a “heliozoan” form (Figure 248).
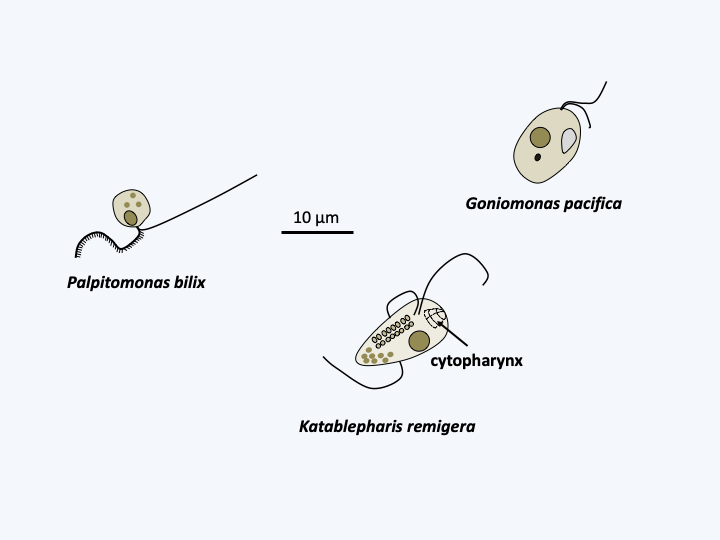
Figure 248.
Some phagotrophic Cryptista.Cryptophyceae
The photosynthetic Cryptista are mostly mixotrophic and form a monophyletic lineage, the class Cryptophyceae. There are about 200 species (Figure 249). These unicellular algae are characterized by the presence of a particular plastid located in the lumen of the reticulum. Indeed, the Rhodophyta which made the endosymbiosis possible has not completely degenerated and it still has a nucleomorph as well as a relic of its endoplasmic reticulum (Box 8). Cryptophyceaen motility is ensured by two flagella decorated with mastigonemes and ejectosomes used to neutralize prey and inserted in a ventral invagination. Mastigonemes are protein extensions attached to the axoneme of certain flagella. Their ontogenesis, structures, presences and arrangements are specific and serve as phylogenetic markers. In Cryptophyceae, they are composed of two parts with distinct diameters and therefore called “bipartite”. They are arranged in two rows on the larger of the two flagella and in one row on the smaller. Among the Hacrobia, mastigonemes are present in several lineages but are bipartite only in Palpitomonas bilix in which they are positioned on only one of the two flagella and on a single row. Cryptophyceae live in fresh and marine waters. They are ubiquitous but prefer cold climates. They constitute an important part of the microflora of ponds in early spring. A few species live in endosymbiosis as a photosynthetic partner with various protozoa. Others have lost the ability to photosynthesize, and instead feed on organic material. Cryptophyceae can sometimes parasitize the digestive tract of animals, in which they are in rare cases responsible for parasitosis. Cryptophyceae did not invent true multicellularity, although some species can form colonies of clumped cells in a gelatinous matrix. They usually reproduce by mitosis and binary fission; the sexual process seems to exist but is poorly understood.
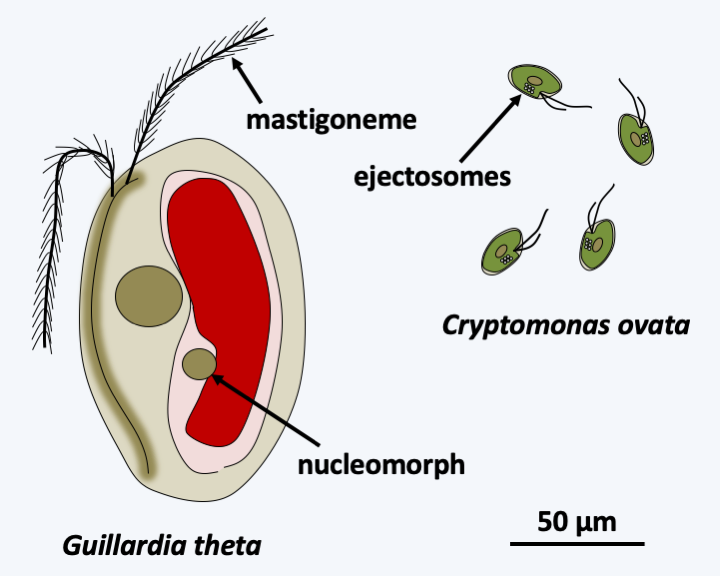
Figure 249.
Two photosynthetic Cryptista.Haptista
The second line of Hacrobia, that of the Haptista, is subdivided into three branches (Figure 247). That of Heliozoa contains the unique class of Centrohelea which groups heliozoans (Figure 250). They are often large, cosmopolitan phagotrophic protozoa that, like other heliozoans, capture their prey using radiating axopods. The microtubules that underlie them have a special arrangement (Figure 251). Most species are covered with scales or spicules, either organic or siliceous, bound by a gelatinous coating. They are able to differentiate cysts and divide by binary fission. Sexual reproduction has not been clearly demonstrated. It could occur in cysts with the differentiation of amoeboid gametes followed by their fusion. Very common in fresh and marine waters, these organisms are able to swallow large prey, including small animals. The Rappemonada phylum contains a few species of algae that are only known by DNA sequences and some fluorescence images. These small algae, about 5 μm in size, have two to four plastids. They seem common and can bloom, especially in the Sargasso Sea. They appear to be related to the Haptophyta which forms the most important branch of the Hacrobia, currently harboring more than 600 species.
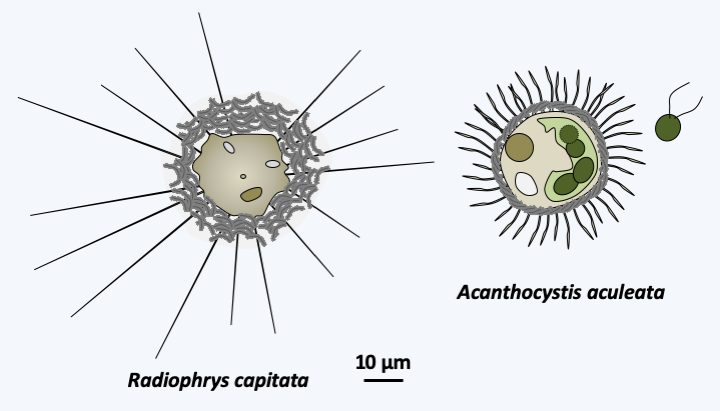
Figure 250.
Two typical centrohelids.
Figure 251.
Arrangement of microtubules in axopods of Radiophrys ambigua.Haptophyta
Haptophyta algae are unicellular, sometimes associated in simple colonies, very small and widespread in all oceans where they form one of the three major groups of phytoplankton, along with diatoms ((Bacillariophyta)) and Dinoflagellata). They therefore play a very important role in the carbon cycle. While most Haptophyta algae inhabit the oceans, a few are terrestrial or inhabit freshwaters. Their diversity is greater in warm seas. They are either mixotrophic or only photosynthetic, more rarely only phagotrophic. They have one or two plastids containing chlorophyll a and c, diadinoxanthins and fucoxanthins. The plastid is surrounded by four membranes, the outermost corresponding to the membrane of the reticulum as in Cryptophyta. On the other hand, if there is any integrated Rhodophyta reticulum, its nucleus has completely disappeared, unlike in Cryptophyta. Haptophytan plastids therefore do not have nucleomorphs. This plastid structure strongly resembles that of the plastids of the Ochrophyta algae of the Stramenopila lineage. The latter, however, do not have the horizontal transfer of the rpl36 gene common to Cryptophyta and Haptophyta. The two flagella of Haptophyta are unequal and bear no ornamentation. All Haptophyta have a structure in common, the haptoneme, which gives the the group its name. This flagellum-like structure is a cytoplasmic extension supported by six or seven microtubules arranged in a semicircle and which functions in the detection and capture of prey (Figure 252). Positioned between the two flagella, the haptoneme varies in size depending on the species and can sometimes only be distinguished at certain stages of the life cycle. Biological characteristics and molecular phylogenies define two classes of Haptophyta (Figure 247).
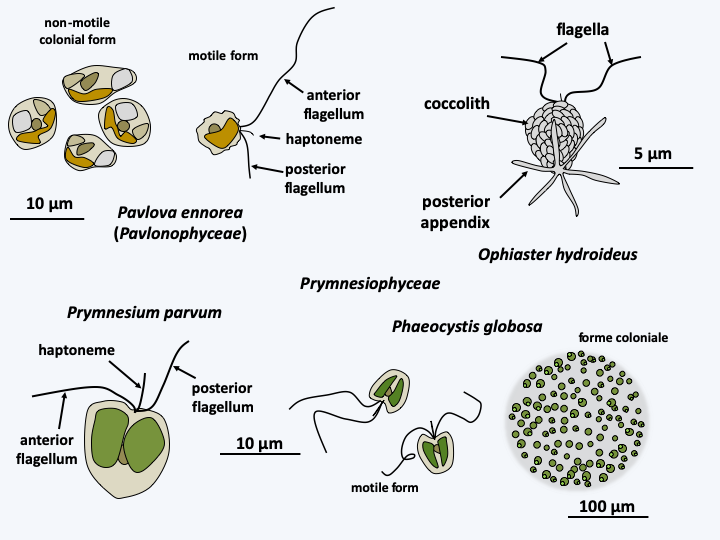
Figure 252.
Diversity of Haptophyta.Pavlophyceae
The Pavlophyceae are known by about fifteen species that are common in coastal or brackish waters, more rarely fresh waters (Figure 252). The plastid is always unique. The two flagella are clearly unequal and the haptoneme anchoring is simple. Unlike most species belonging to the other class, that of Prymnesiophyceae, they have no scales. This class is very diverse as it contains most of the 600 known Haptophyta species (Figure 252).
Prymnesiophyceae
In Prymnesiophyceae, the number of plastids varies between one and two and the flagella are very slightly unequal. Prymnesiophyceae cells are often covered with organic plaques, which can calcify or more rarely silicify. The whole thing is wrapped in a mucilage. The plaques are formed from the Golgi apparatus and are called coccoliths if they are calcified. The best-known present-day representative who serves as a model for the Haptophyta is Emiliania huxleyi (Figure 253). It belongs to the order of Coccolithales, whose representatives always differentiate coccoliths during one of the stages of their life cycle. The life cycle has been well studied in Emiliania huxleyi, in whom it is of the haplodiplobiontic type. It consists of two stages very different morphologies: a swimming haploid stage and a non-motile diploid stage during which the cell is surrounded by coccoliths (Figure 253). Analysis of genomes from various strains shows unexpected coding capacity with more than 30,000 genes spread over 140 Mb. It also reveals a great genetic variability, suggesting that the morphospecies Emiliania huxleyi hides several true species.
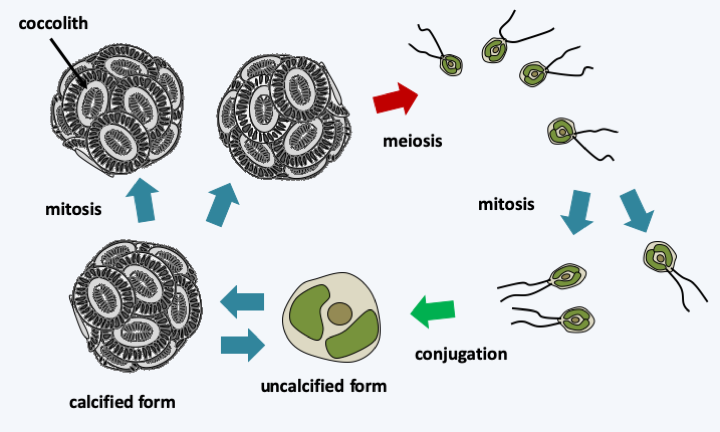
Figure 253.
Cycle of Emiliania huxleyi. Haploid cells are small, motile, and lack coccoliths. Their conjugation leads to large diploids, non-motile and covered with coccoliths. It is possible to find naked cells in cultures of calcified cells.Besides extant species, there are many known fossil Haptophyta, because their coccoliths are easily petrified. The earliest date back to 300 Ma ago. Haptophytes were particularly abundant in the Jurassic and especially in the Cretaceous, because they form a good part of the sedimentary deposits dating from these periods. Then, they seem to have suffered a mass extinction.
Back to chapter index